Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 -butyraldehyde in tributyl phosphate diluted with
-butyraldehyde in tributyl phosphate diluted with  -dodecane
-dodecaneBan, Yasutoshi; Asakura, Toshihide; Morita, Yasuji
Radiochimica Acta, 92(12), p.883 - 887, 2004/12
Times Cited Count:10 Percentile:55.63(Chemistry, Inorganic & Nuclear)The reduction kinetics of Np(VI) by  -butyraldehyde in 30%TBP solution diluted with
-butyraldehyde in 30%TBP solution diluted with  -dodecane were analyzed by spectrophotometry. Based on the results of both
-dodecane were analyzed by spectrophotometry. Based on the results of both  -butyraldehyde and nitric acid concentration dependencies on the Np(VI) reduction reaction, the rate equation was obtained as -d
-butyraldehyde and nitric acid concentration dependencies on the Np(VI) reduction reaction, the rate equation was obtained as -d [Np(VI)]/d
[Np(VI)]/d =
= 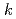 [
[ -C
-C H
H CHO]
CHO]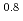 [HNO
[HNO ]
]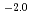 [Np(VI)]
[Np(VI)] where
where 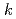 = (1.0
= (1.0 0.2)
0.2)  10
10 M
M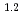 min
min at 294
at 294  1 K. The activation energy of the reaction was 76
1 K. The activation energy of the reaction was 76  5 kJ/mol. Redox equilibrium between Np(V) and N(VI), and reaction mechanism were discussed.
5 kJ/mol. Redox equilibrium between Np(V) and N(VI), and reaction mechanism were discussed.
Uchiyama, Gunzo; Hotoku, Shinobu; Fujine, Sachio; Maeda, Mitsuru
JAERI-M 93-198, 27 Pages, 1993/10
no abstracts in English